1. Introduction
Lime is produced by heating limestone which is more or less pure calcium carbonate. During heating, carbon dioxide is driven off and non-volatile impurities such as Oxides of Silicon, Magnesium, Aluminium, Iron and Manganese are left behind in the lime.

Pure calcium oxide is a white solid which melts at 2570°C. When the molten material is cooled, it solidifies in cubic crystals, but as ordinarily prepared it is non-crystalline, easily powdered and has a specific gravity varying from 3.08 to 3.30.
The specific gravity of lime depends on the temperature to which it has been heated, higher temperatures giving a denser product.
2. Sources of Lime
The lime is not generally found in nature in the free state but it is obtained by burning one of the following materials:
a. Limestone found in limestone hills
b. Limestone builders found in the beds of old rivers
c. Kankar Found below ground
d. Shells of sea animals.
3. Properties of Lime
Following are the properties of good quality lime, which makes it suitable for use as an engineering material
a. Easily workable.
b. Provides strength to the masonry.
c. Posses good plasticity.
d. Offers good resistance to moisture.
e. Stiffens early.
f. An excellent cement and adheres to the masonry units perfectly.
g. Lime masonry provides durability due to low shrinkage in drying.
| Read Also: Lime Plaster |
4. Uses of Lime
Followings are the various uses of lime:
a. It is used as a matrix for concrete.
b. It is used as a binding material in mortars for stoneware and also in bedding and jointing brickwork of low strength.
c. It is used for plastering walls, ceilings, etc.
d. It is employed for whitewashing and as a base coat for distempers.
e. It is used for knotting of timber work before painting.
f. It is used for the production of artificial stone, lime-sand bricks, foam-silicate products, etc.
g. When mixed with portland cement, the lime-cement mortar attains such valuable properties that it replaces the costly cement plaster and serves as a plasticiser.
h. It is used as a flux in the manufacture of steel.
i. Eminently hydraulic lime can be used for masonry work below ground.
j. It is used in the manufacture of paints.
k. It is used for stabilizing the soils.
l. It is employed for creating good sanitary conditions in foul, damp and filthy places.
5. Constituents of Limestones
Following are the constituents of limestones ( Main source of getting lime ) :
Clay:
a. It is responsible for producing hydraulicity in lime.
b. It retards slaking when present in small quantity, arrests slaking. When it is in excess.
c. It makes lime insoluble in water.
d. To make a good lime it is desirable to have 8 to 30% of clay.
Soluble silica:
a. To develop hydraulicity it is imperative to have silica and alumina present in chemical combination with limestone.
Hydraulicity is caused due to silicates of calcium, magnesium and aluminium.
These silicates are inserted at low temperatures but they become active and combine with lime at high temperatures.
Magnesium carbonate:
a. It increases the setting process but reduces slaking.
b. Lime containing not more than 5% of magnesium carbonate gives better results and imparts hydraulics properties to the lime. But limes containing large proportions of magnesium carbonate are liable to crack (which ultimately disintegrate).
c. When the content of magnesium carbonate is about 30%, the hydraulicity is rendered to the lime even in absence of clay.
Alkalies and metallic oxides:
They tend to become soluble silicates at low temperatures only and thus cause hydraulicity.
Sulphates:
They tend to reduce slaking but increase setting action only when they are not in excess.
Iron Compounds:
The iron compounds if present, lower the temperature of calcination of limestone.
Carbonaceous matter:
The presence of carbonaceous matter is harmful; it produces a very poor quality lime.
6. Types of Lime
A. Types of Lime Based on obtained by calcining limestones
Limes obtained by calcining limestones are broadly classified as follows:
i. Flat Lime
ii. Hydraulic Lime
iii. Poor Lime
i. Flat Lime
a. It is also known as rich, flat, common, high calcium, air or pure lime.
b. It is also called (Fat lime) as it slakes vigorously and its volume is increased to about 2 to 2.5 times the volume of quick lime.
c. It is prepared by calcining comparatively pure carbonate of lime which contains about 95% of calcium oxide.
Properties:
1. Slake Vigorously
2. Possess a high degree of plasticity
3. Hardens very slowly
4. Possess perfectly white colour
5. Sets slowly in presence of air
6. Soluble in water which is changed frequently
Uses:
1. It is used to whitewash and plaster on walls.
2. Lime mortar, made of lime and sand is used for thin joints of brickwork and stonework.
3. Lime mortar, made of lime and surkhi (powder obtained by grinding of burnt bricks) may be used for thick masonry walls, foundations, etc.
ii. Hydraulic Lime:
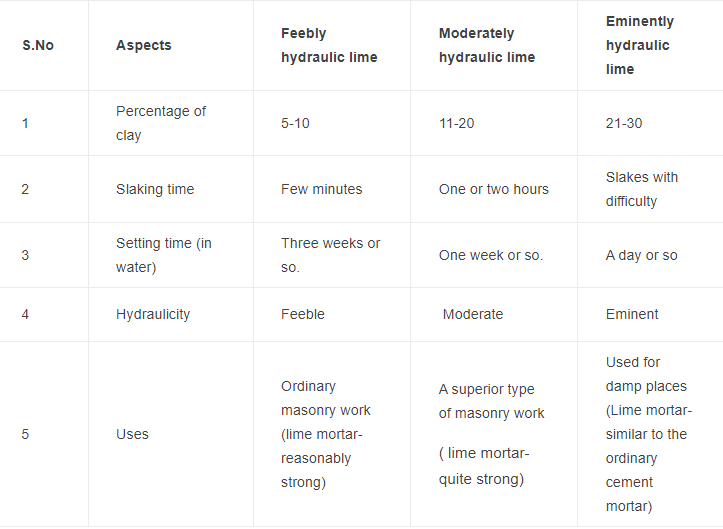
1. It is also known as water lime. The hydraulic lime, depending upon the percentage of clay is divided into the following three types:
(i) Feebly Hydraulic lime
(ii) Moderately hydraulic lime
(iii) Eminenetly Hydraulic lime
The increase in the percentage of clay makes the slaking difficult and increases the hydraulic property.
2. It can be set underwater and in thick walls where there is no free circulation of air.
3. It forms a thin paste with water.
4. It is not perfectly white in colour, as such it appears less sanitary than the flat lime.
5. The hydraulic lime containing about 30% of clay resembles natural cement.
Table1 shows the comparison between various types of hydraulic limes.
| Read Also: Types of Pipes in Civil Engineering & Construction |
iii. Poor Lime:
1. It is also known as lean or meagre.
2. It contains more than 30% of clay.
3. Its colour is muddy white.
4. It slakes very slowly.
5. It forms a thin paste with water.
6. It sets/hardens very slowly.
7. It has poor binding properties.
Uses:
As the mortar made from this type of lime is of poor quality, therefore, such mortar is employed for the inferior type of work.
B. Classification of Building limes according to I.S.I Specifications:
According to Indian standard specifications, lime is classified as follows:
1. Class A: Eminently hydraulic lime – used for structural purposes.
2. Class B: Semi-hydraulic lime – used for masonry mortars.
3. Class C: Fat Lime – used for finishing coat of plastering, whitewashing, etc. and with the addition of pozzolanic material for masonry mortar.
4. Class D: Magnesium lime – used for finishing coat in plastering, whitewashing, etc.
5. Class E: Kankar Lime
6. Class F: Kankar lime – used for masonry mortars.
| Read Also: Parapet Wall |
| Read Also: Reinforced Cement Concrete (RCC) |

